Navigation
What is DOTAD
In comparison to small molecules, antibodies have several advantages such as high specificity, efficacy, safety, and longer half-life, making them indispensable in the treatment of complex systemic diseases. Since the approval of the first antibody drug OKT3 (muromonab-CD3) by the FDA in 1986, the field of antibody development has experienced rapid growth. As of the end of June 2023, the FDA has approved 119 antibody drugs, which are widely used for prevention, diagnosis, and treatment of severe diseases.
Jain et al. conducted a comprehensive assessment of the biophysical and physicochemical characteristics between candidate antibodies in Phase Ⅱ or Ⅲ clinical trials and FDA-approved antibody drugs, revealing significant risks associated with the developability of candidate drugs in Phase Ⅱ or Ⅲ. Apart from binding to the target molecule, antibody drugs must also meet a range of biophysical and physicochemical criteria that reflect their manufacturability, safety, and other factors collectively known as developability.Although there are several databases dedicated to therapeutic antibodies, they have not been specifically designed for antibody developability assessment and lack the necessary biophysical and physicochemical properties required for such assessments.
DOTAD is the first all-inclusive database of therapeutic antibody development information available on the market, providing a platform for storing, browsing, and searching for therapeutic antibody items that are accessible for research and development. Furthermore, DOTAD incorporates cutting-edge resources for the evaluation of antibody developability, providing a comprehensive platform for researchers and companies in need.
Fields in DOTAD
The therapeutic metadata within DOTAD serves as a comprehensive classification system for therapeutic antibodies, enabling users to swiftly locate specific therapeutic antibodies based on their desired criteria. It encompasses crucial information pertaining to the source of the antibody sequence, identification of target molecules and receptors, antibody format, heavy chain class (HC_Class), light chain type (LC_Type) and valuable clinical trial information related to diverse therapeutic regimens. In addition, the metadata includes the PDB_id, which provides information on the antibody structure, and the detection rate of anti-drug antibodies that react to immunogenicity.
Each sequence is identified by a specific DOTAD_ID, with each ID uniquely representing a single sequence. The INN, established by the World Health Organization, is used to name each sequence if applicable. Antibodies generated in animal models may elicit an immune response in humans due to their immunogenicity, which could limit their application[24]. The "Species" field in the metadata records the species origin of the antibody, including human, mouse, rabbit, etc. The species origin can impact the antibody's specificity, affinity, and immunogenicity. For certain drugs that enter the human body, they may induce the immune system to produce Anti-Drug Antibodies (ADAs). In some cases, these antibodies can affect the efficacy, safety, and tolerability of the drug. Therefore, ADAs can also be used to assess and evaluate the immunogenicity and safety of drugs. The "ADAs_rate" refers to the detection rate of ADAs, which indicates the proportion of patients receiving a certain therapeutic drug who develop antibodies against the drug. A high ADAs_rate may suggest a risk of autoimmune reactions associated with the drug, which could potentially reduce therapeutic effectiveness or cause adverse reactions. The "HC_Class" of an antibody refers to the specific class or subclass of its heavy chains. In humans, commonly observed antibody heavy chain isotypes include IgG1, IgG2, IgG3, IgG4, IgM, IgA1, IgA2, IgD and IgE. The "HC_Class" of an antibody dictates the structure and function of its heavy chain. The choice of "HC_Class" impacts the architecture and functional characteristics of the antibody. It has been demonstrated that the process of immunoglobulin class switch and transition to high-affinity IgG1 and IgG4 antibodies is associated with diminished drug efficacy, increased clearance rate, generation of neutralizing antibodies, and modulation of hypersensitivity reaction rates. Therefore, the selection of appropriate "HC_Class" plays a pivotal role in the design, development, and application of specific antibodies. The "LC_Type" consists of two types in mammals: kappa chain (κ chain) and lambda chain (λ chain). Different types of light chains not only exhibit subtle structural differences in antibodies but also potentially impact the immune function and conformational properties of these molecules. Studies have indicated that the "elbow" angle of an antibody can be influenced by its light chain types[28], and the inherent flexibility of the "elbow" region may introduce disorder, thereby diminishing the efficacy of the antibody[29]. Therefore, meticulous selection and optimization of the light chain are pivotal considerations in antibody engineering and therapeutic antibody development. Antibodies possess high specificity and can interact with specific target structures or molecules. The "Targets" column refers to the target substance or molecule that the antibody is intended to bind to, including antigens, receptors, and intracellular molecules. Based on different targets, antibodies can be designed to inhibit pathogen infections, regulate immune responses, diagnose and treat diseases, among other applications. Clinical trials are critical steps in the drug development process, used to evaluate the safety and efficacy of drugs. The "Highest_Clin_Trial" column indicates the highest stage of clinical trial currently being conducted for the antibody. The "PDB" column displays the PDB number of the antibody, which can be clicked on to view the specific structural information of the antibody.
This section provides a comprehensive compilation of experimentally measured and computed biophysical and physicochemical properties that are critical for assessing the developability of antibodies. The included experiments encompass a range of techniques such as CIC, PSR binding assay, CSI-BLI, AC-SINS, SGAC-SINS, Tm determination using differential scanning fluorescence (DSF), SMAC, accelerated stability SEC slope, HIC, BVP assay, and ELISA. For a succinct summary of these experimental assays, please refer to the table below.
| Assay | Description | Reference |
|---|---|---|
| SGAC-SINS | Self-interaction measured using AC-SINS in the presence of ammonium sulfate | [1] |
| HIC | Hydrophobic interaction chromatography retention time | [2] |
| SMAC | Standup monolayer adsorption chromatography | [3] |
| Tm | Melting temperature | [4] |
| AS | Accelerated stability measured by SEC% monomer | |
| ELISA | Average of binding to insulin, ssDNA, dsDNA, cardiolipin, KLH, LPS | [5] |
| BVP | Binding to baculovirus particle in ELISA | [6] |
| DNP | Binding to 2,4-dinitrophenol in ELISA | [7] |
| PSR | Binding to polyspecificity reagent | [8] |
| CIC | Cross interaction chromatography | [9] |
| ACSINS | Self interaction | [10] |
| CSI | Self interaction | [11] |
| FcRn Rel RT | Retention time on FcRn column | [12] |
| Hep RT | Retention time on heparin column | |
| Fe+2 FVIII | Acquired FVIII binding upon exposure to ferrous ions | [13] |
| Fe+2 C3 | Acquired C3 binding upon exposure to ferrous ions | |
| Fe+2 LysM | Acquired LysM binding upon exposure to ferrous ions | |
| Heme FVIII | Acquired FVIII binding upon exposure to heme | |
| Heme C3 | Acquired C3 binding upon exposure to heme | |
| Heme LysM | Acquired LysM binding upon exposure to heme | |
| Heme | Binding to Heme in ELISA | |
| FA | Binding to folate in ELISA |
How to use DOTAD
Search
On the Search page of DOTAD, users can specify one or more fields to perform a quick search with the keyword(s) in the field(s). Users can simply click example and submit buttons to perform an example search.
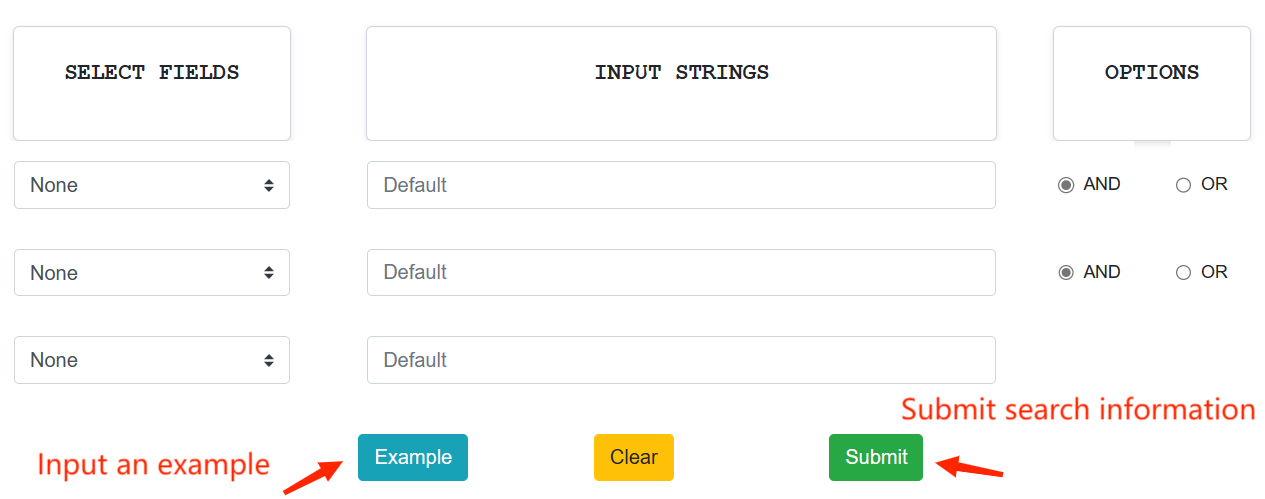
Browse
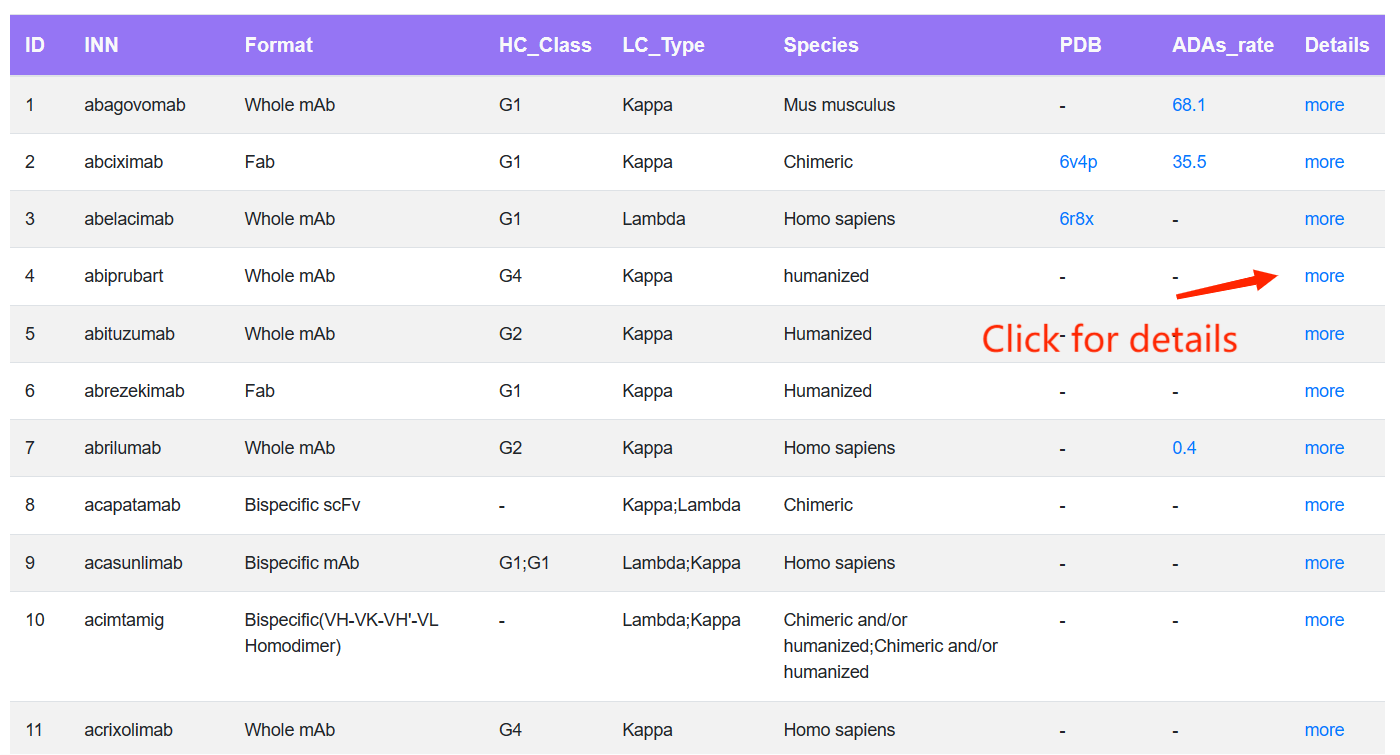
On the Browse page, users can click one term to view the specified data in DOTAD. Users initially access a concise browsing table that presents fields such as INN name, target, mAb isotype, LC class, clinical status, biophysical and physicochemical properties, and links directing to the "Detail" page.
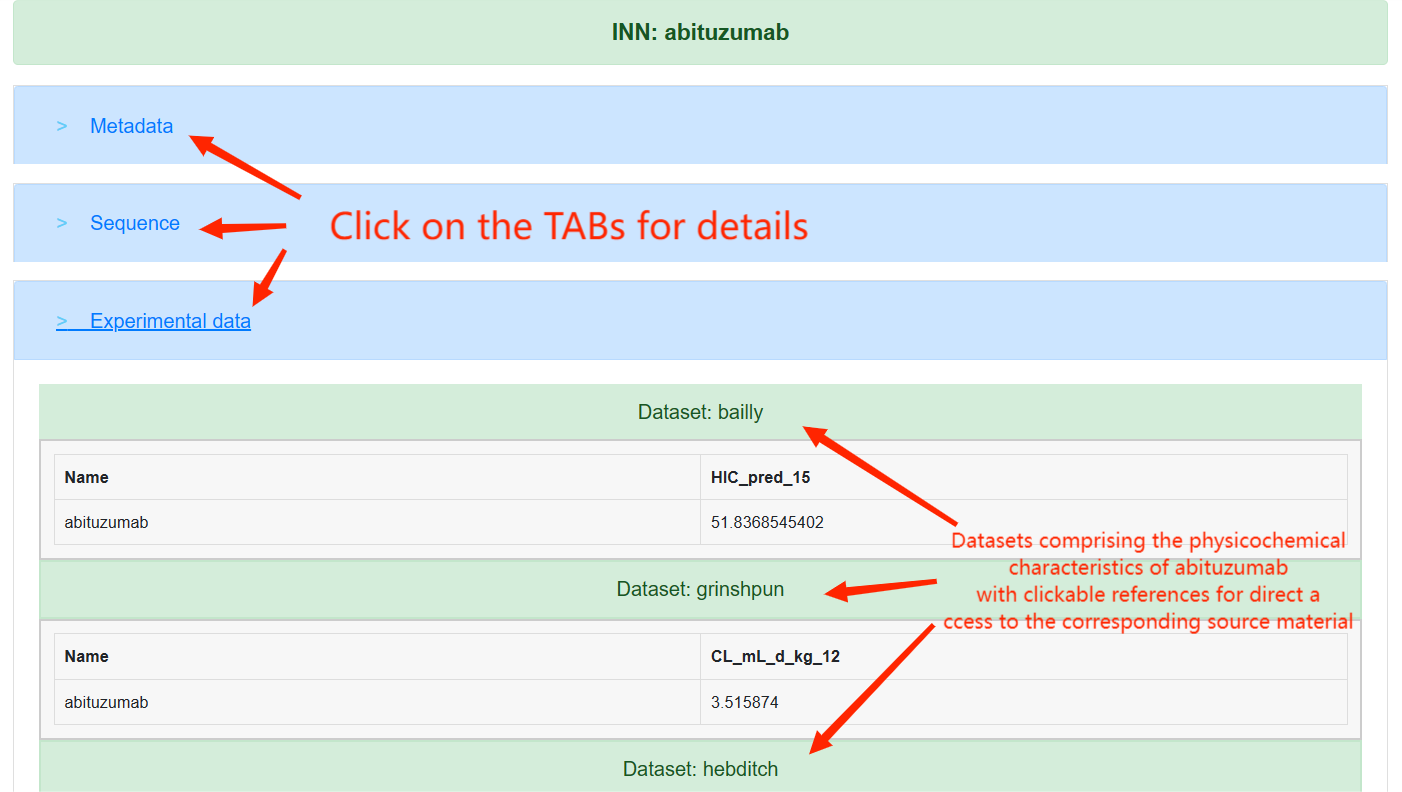
On the Details page, users can access specific information regarding a therapeutic antibody, including therapeutic metadata, sequence data, and developability information. By selecting the "Developability Data" tab, users will be able to view detailed biophysical and physicochemical properties of the antibody as measured by various experiments.
Download
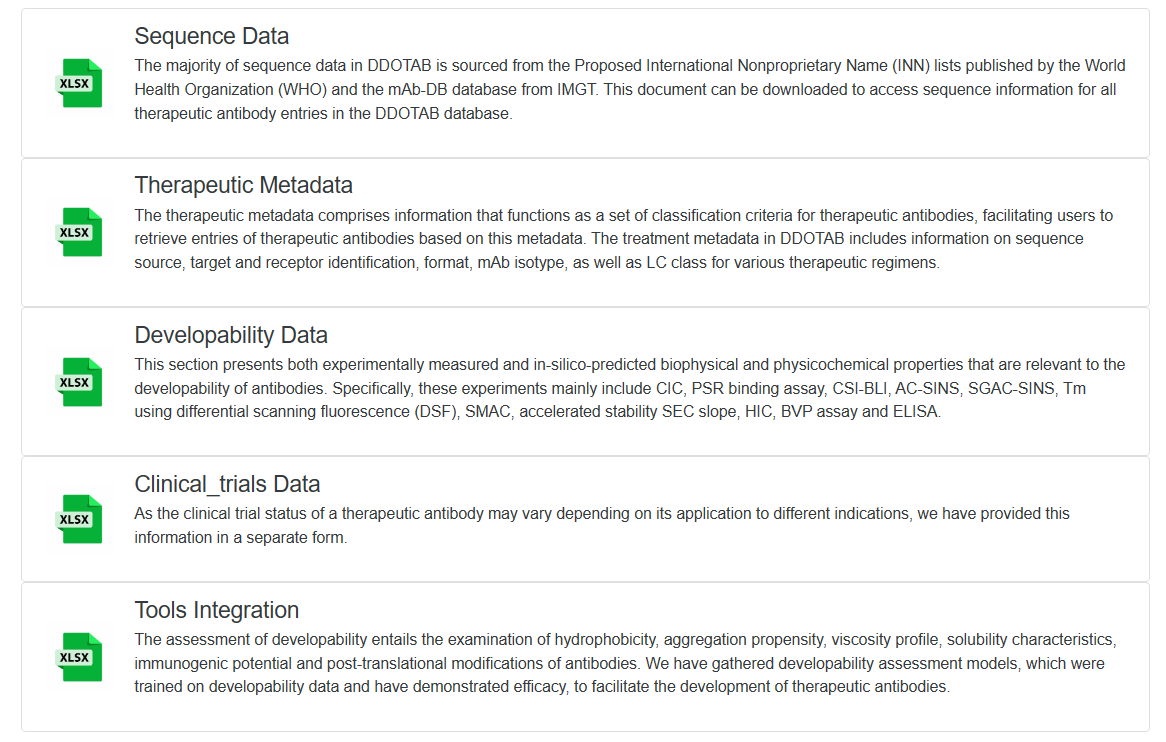
We have categorized the data and tools in DOTAD into five distinct tables for easy access and analysis. Users can conveniently download all the information from our dedicated download page.
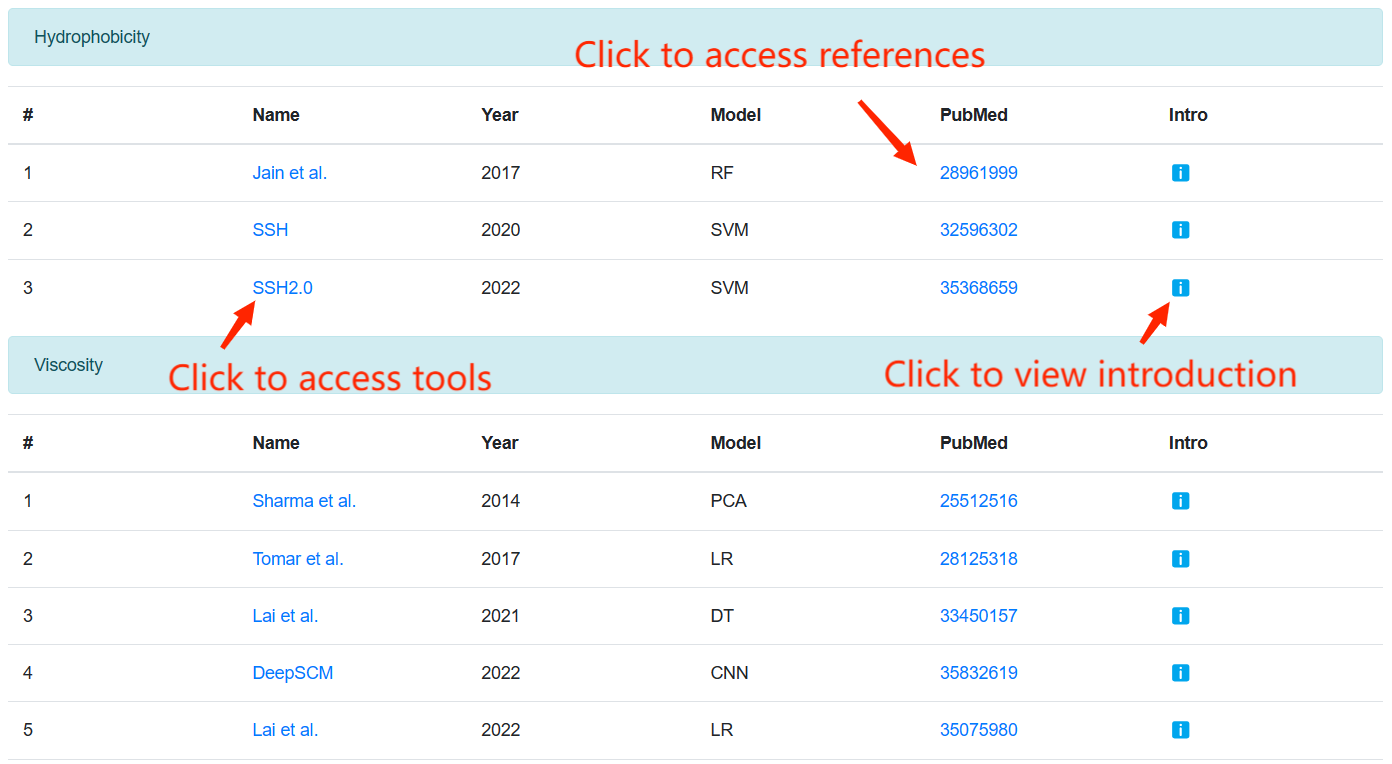
Tools Integration
This page integrates state-of-the-art tools for predicting the developability of therapeutic antibodies. Users can access the corresponding tool by clicking on its name or refer to the relevant reference by clicking on "reference".
Contact Us
Are You Facing Any Problem..?
DOTAD has been developed and maintained by HLAB. Like any other database, errors may have occurred during the data accumulation phase. If you come across any errors in the database or find any published motifs/sequences that are unrelated to the target but not included in the database, please inform us. We welcome your feedback and it will greatly assist us in improving DOTAD for the scientific community engaged in basic and applied research. Should you have any other problems or questions, please don't hesitate to contact us.
Please contact us at