Details
Structure visualisation
Entry information
| Complex | |
| AACDB_ID: | 476 |
| PDBID: | 3ETB |
| Chains: | I_M |
| Organism: | Mus musculus, Bacillus anthracis |
| Method: | XRD |
| Resolution (Å): | 3.80 |
| Reference: | 10.1016/j.jmb.2009.02.003 |
| Antibody | |
| Antibody: | M18 scFv |
| Antibody mutation: | No |
| INN (Clinical Trial): | |
| Antigen | |
| Antigen: | anthrax protective antigen domain 4 |
| Antigen mutation: | No |
| Durg Target: | P13423 |
Sequence information
Antibody
Chain: I
Mutation: NULL
| >3ETB_I|Chain A[auth F], B[auth G], C[auth H], D[auth I]|Antibody M18 light chain and antibody M18 heavy chain linked with a synthetic (GGGGS)4 linker|Mus musculus (10090) MADYKDIQMTQTTSSLSASLGDRVTVSCRASQDIRNYLNWYQQKPDGTVKFLIYYTSRLQPGVPSRFSGSGSGTDYSLTINNLEQEDIGTYFCQQGNTPPWTFGGGTKLEIKRGGGGSGGGGSGGGGSGGGGSEVQLQQSGPELVKPGASVKISCKDSGYAFNSSWMNWVKQRPGQGLEWIGRIYPGDGDSNYNGKFEGKAILTADKSSSTAYMQLSSLTSVDSAVYFCARSGLLRYAMDYWGQGTSVTVSS |
Antigen
Chain: M
Mutation: NULL
| >3ETB_M|Chain E[auth J], F[auth K], G[auth L], H[auth M]|Anthrax Protective Antigen|Bacillus anthracis (1392) RDKRFHYDRNNIAVGADESVVKEAHREVINSSTEGLLLNIDKDIRKILSGYIVEIEDTEGLKEVINDRYDMLNISSLRQDGKTFIDFKKYNDKLPLYISNPNYKVNVYAVTKENTIINPSENGDTSTNGIKKILIFSKKGYEIG |
Interaction
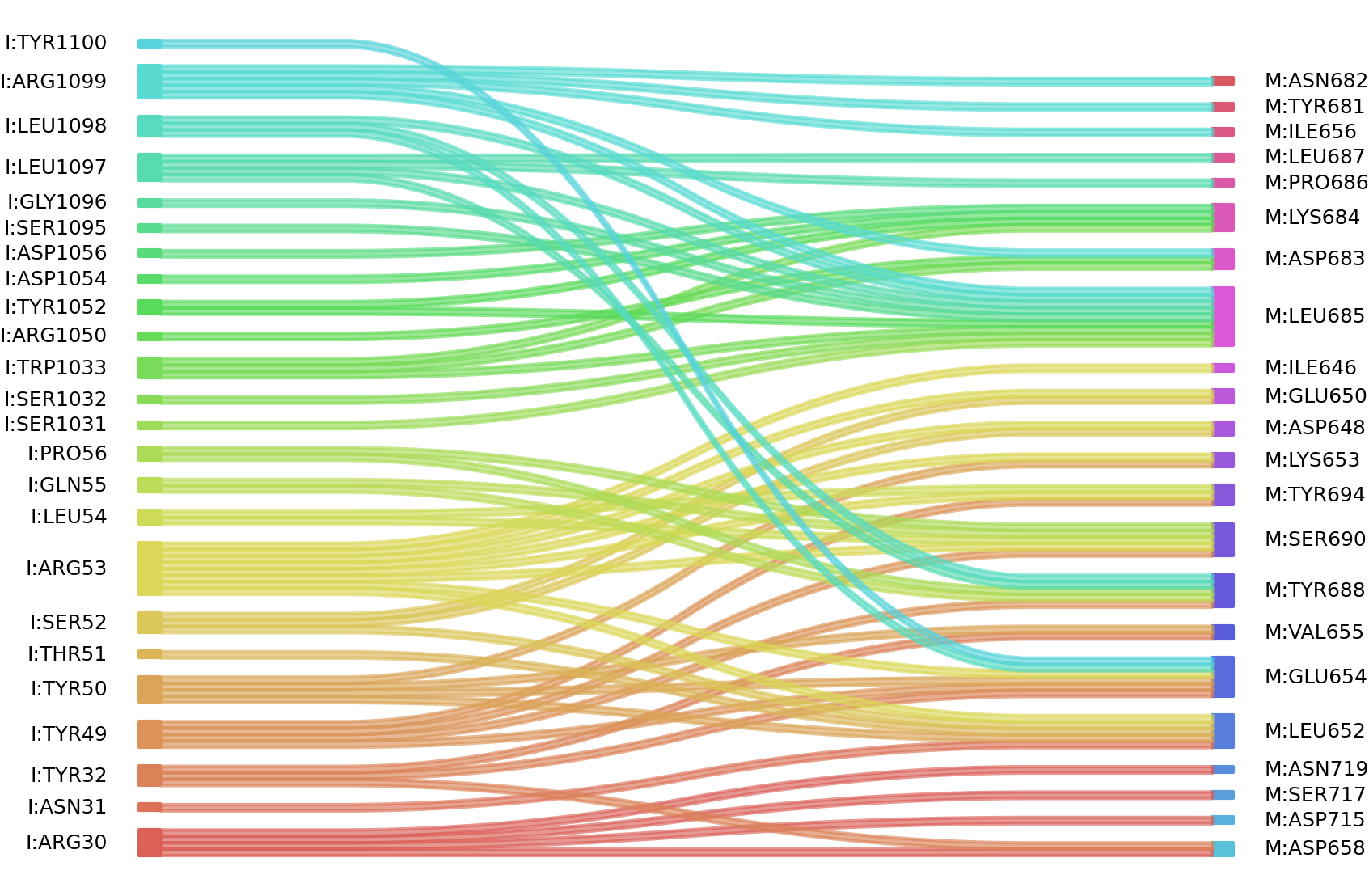
1、Solvent accessible surface areas (SASA) were calculated (Naccess V2.1.1) for each residue in antibody and antigen, respectively. The residues with SASA loss in binding of more than 1Å2 were classified as interacting residues.
Interacting residues (ΔSASA based)
| Chain residues position delta_SASA
: residuesposition I: ARG30 ASN31 TYR32 PHE46 TYR49 TYR50 THR51 SER52 ARG53 LEU54 GLN55 PRO56 SER1031 SER1032 TRP1033 ARG1050 ASP1054 ASP1056 SER1095 LEU1097 LEU1098 ARG1099 M: ILE646 ASP648 GLU650 LEU652 LYS653 GLU654 VAL655 ILE656 ASP658 MET662 LYS679 TYR681 ASN682 ASP683 LYS684 LEU685 PRO686 LEU687 TYR688 SER690 TYR694 ASP715 SER717 ASN719 |
2、We defined interacting paratope-epitope residues by a distance cutoff of < 6 Å . Two amino acids are considered as interacting residues if they have at least one atom within a distance of 6 Å from any atom.
Interacting residues (Atom distance based)