Details
Structure visualisation
Entry information
| Complex | |
| AACDB_ID: | 204 |
| PDBID: | 1ZTX |
| Chains: | HL_E |
| Organism: | West Nile virus, Mus musculus |
| Method: | XRD |
| Resolution (Å): | 2.50 |
| Reference: | 10.1038/nature03956 |
| Antibody | |
| Antibody: | E16 Fab |
| Antibody mutation: | No |
| INN (Clinical Trial): | |
| Antigen | |
| Antigen: | West Nile Virus Envelope Protein DIII |
| Antigen mutation: | No |
| Durg Target: | |
Sequence information
Antibody
Heavy Chain: H
Mutation: NULL
| >1ZTX_H|Chain B[auth H]|Heavy Chain of E16 Antibody|Mus musculus (10090) QVQLQQSGSELMKPGASVQISCKATGYTFSDYWIEWVKQRPGHGLEWIGDILCGTGRTRYNEKLKAMATFTADTSSNTAFMQLSSLTSEDSAVYYCARSASYGDYADYWGHGTTLTVSSAKTTPPSVYPLAPGCGDTTGSSVTLGCLVKGYFPESVTVTWNSGSLSSSVHTFPALLQSGLYTMSSSVTVPSSTWPSQTVTCSVAHPASSTTVDKKLEPS |
Light Chain: L
Mutation: NULL
| >1ZTX_L|Chain C[auth L]|Light Chain of E16 Antibody|Mus musculus (10090) DIVMTQSHKFMSTSVGDRVSITCKASQDVSTAVAWYQQKPGQSPKLLISWASTRHTGVPDRFTGSGSGTDYTLTISSVQAEDLALYYCQQHYTTPLTFGAGTKLELKRADAAPTVSIFPPSSEQLTSGGASVVCFLNNFYPKDINVKWKIDGSERQNGVLNSWTDQDSKDSTYSMSSTLTLTKDEYERHNSYTCEATHKTSTSPIVKSFNRN |
Antigen
Chain: E
Mutation: NULL
| >1ZTX_E|Chain A[auth E]|Envelope protein|West Nile virus (11082) GSQLKGTTYGVCSKAFKFLGTPADTGHGTVVLELQYTGTDGPCKVPISSVASLNDLTPVGRLVTVNPFVSVATANAKVLIELEPPFGDSYIVVGRGEQQINHHWHKSG |
Interaction
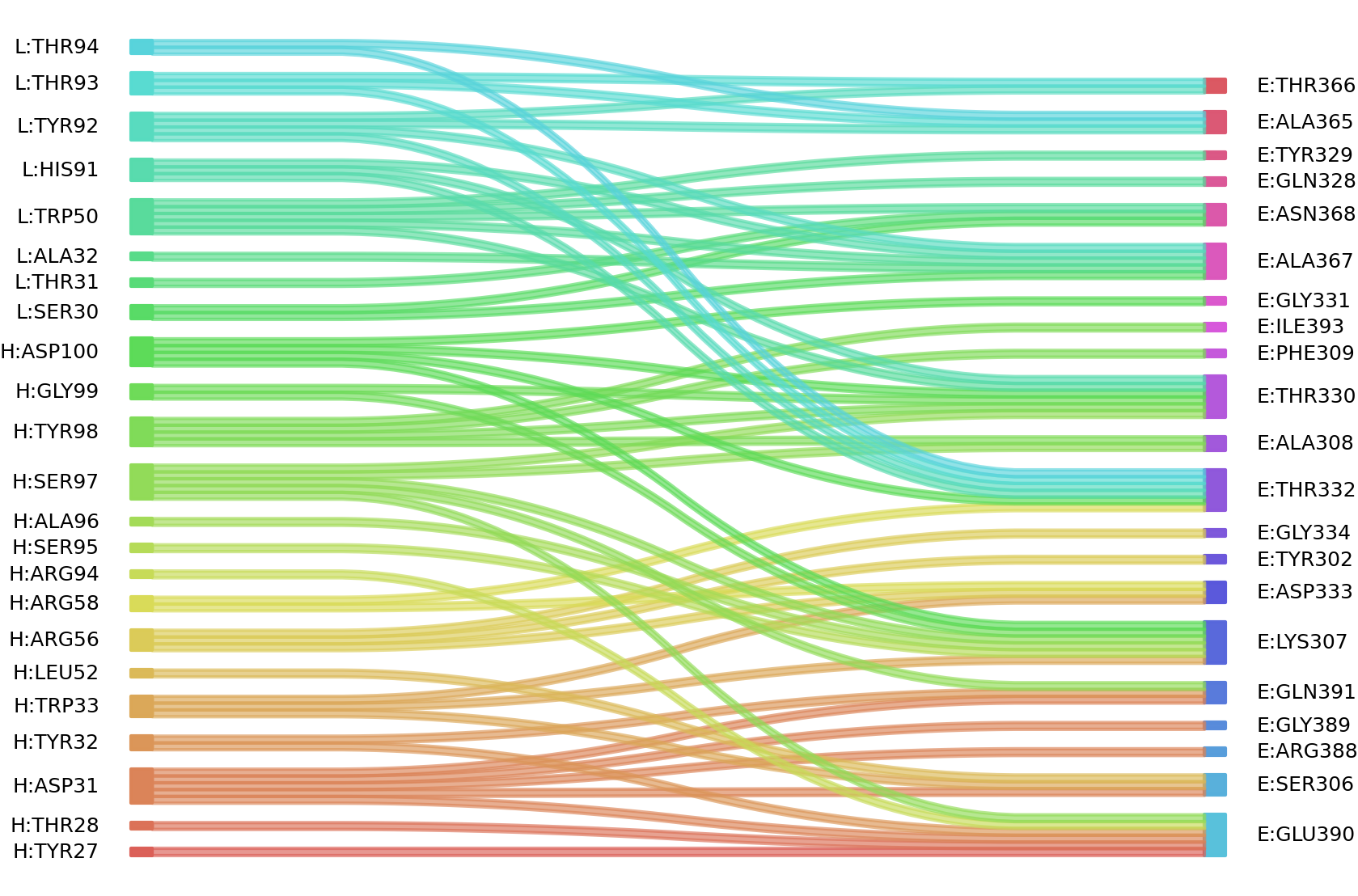
1、Solvent accessible surface areas (SASA) were calculated (Naccess V2.1.1) for each residue in antibody and antigen, respectively. The residues with SASA loss in binding of more than 1Å2 were classified as interacting residues.
Interacting residues (ΔSASA based)
| Chain residues position delta_SASA
: residuesposition H: TYR27 THR28 ASP31 TYR32 TRP33 ARG56 ARG58 ARG94 SER95 ALA96 SER97 TYR98 GLY99 L: SER30 THR31 ALA32 TRP50 HIS91 TYR92 THR93 THR94 E: TYR302 GLY303 VAL304 SER306 LYS307 ALA308 PHE309 LYS310 GLN328 THR330 GLY331 THR332 ASP333 ALA365 THR366 ALA367 ASN368 GLY389 GLU390 GLN391 ILE393 |
2、We defined interacting paratope-epitope residues by a distance cutoff of < 6 Å . Two amino acids are considered as interacting residues if they have at least one atom within a distance of 6 Å from any atom.
Interacting residues (Atom distance based)